TRANSPARENT ANALYTICS.
Rapid for Trials streamlines clinical trial screening and enrollment workflow. A feature within the Rapid mobile app, Rapid for Trials delivers results from Rapid clinical modules to study team members’ mobile devices, streamlining communications, maximizing screening potential and reducing time to enrollment.
The Rapid platform has been used to select patients and conduct core lab analysis for some of the most influential stroke clinical trials ever performed – all 6 randomized trials had positive results and published in the NEJM. RapidAI for Trials offers unparalleled clinical trial expertise.
.png?width=397&height=859&name=CHARM-Notification-Aspects@3x%20(4).png)
.png?width=1125&height=2436&name=CHARM-Notification-Patient-Card@3x%20(2).png)
.png?width=1125&height=2436&name=CHARM-Patient-Images@3x%20(2).png)
-1.png?width=1125&height=2436&name=CHARM-Notification-Patient-Status%20(2)-1.png)
Alerts are sent immediately to the research team when potential study candidates are identified – with customizable alert configurations.
Analytics deliver transparency of potential patient volumes at participating sites.
Study team members are provided access to source imaging data as well as clinical data.
Study team members are provided access to source imaging data, as well as clinical data.
Rapid for Trials helps identify relevant patients–enhancing the volume and quality of trial registrants.
Rapid offers a suite of modules to identify patients that meet study inclusion and exclusion criteria.
Automated quantification of eligibility criteria is available immediately to improve coordinator speed and efficiency while screening patients for enrollment.
Get site-level visibility into scan volume based on site specific inclusion and exclusion criteria.
The Rapid platform has been used in the most influential stroke clinical trials in the world for both patient selection and core lab analysis.
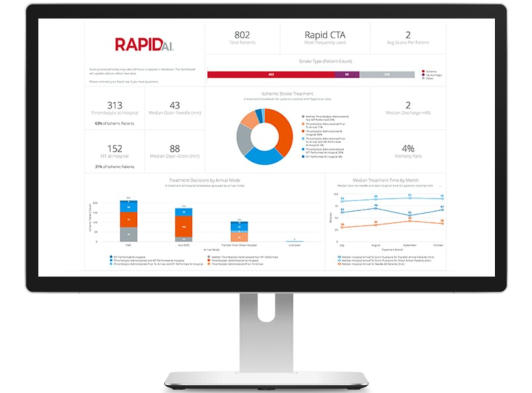
The RapidAI Insights product provides a clinical trial study dashboard to aid in tracking site-level performance, such as number of scans per site and number of potential patients identified based on each of the modules. Dashboards can be customized to the unique requirement of trial sponsors.
“ We used RAPID in the EXTEND, EXTEND-IA and EXTEND-IA TNK randomized trials at over 20 sites across Australia and New Zealand. It’s straightforward to install and works with the full range of CT and MR devices and PACS systems. The fully automated results are robust to common artefacts, easily interpretable and provide standardization across sites with a range of imaging experience.”


