Become Part of the RapidAI Revolution
If you are interested in learning more about including RapidAI™ in a clinical study, Contact Us.

After initial validation in an NIH-sponsored DEFUSE 2 study, published in 2012, and extensively studied in 14 worldwide clinical trials, iSchemaView purchased the technology rights to the software that same year. Since then, adoption of the Rapid® platform has quickly grown.
In early 2019, iSchemaView received FDA clearance that allows physicians to use our neuroimaging platform to select stroke patients likely to benefit from endovascular thrombectomy (clot removal). We believe this advancement will benefit smaller community hospitals and the patients they serve.

Considered the preeminent brain imaging platform, we also have a highly experienced leadership team. Co-founders Dr. Greg Albers and Dr. Roland Bammer, along with software engineer Matus Straka are recognized leaders in cerebrovascular neuroscience, cerebrovascular radiology, and software engineering. Our mission is to continue to redefine and improve stroke care, providing healthcare organizations with the fastest, most advanced technology to facilitate the diagnosis and treatment of stroke.

If you are interested in learning more about including RapidAI™ in a clinical study, Contact Us.
Find out about the most influential stroke clinical trials taking place worldwide. The objective, status, design, endpoint.
See All Clinical Trials.
From early to late-window results, RapidAI has achieved the best results ever obtained in endovascular stroke studies.
See All Trial Results.
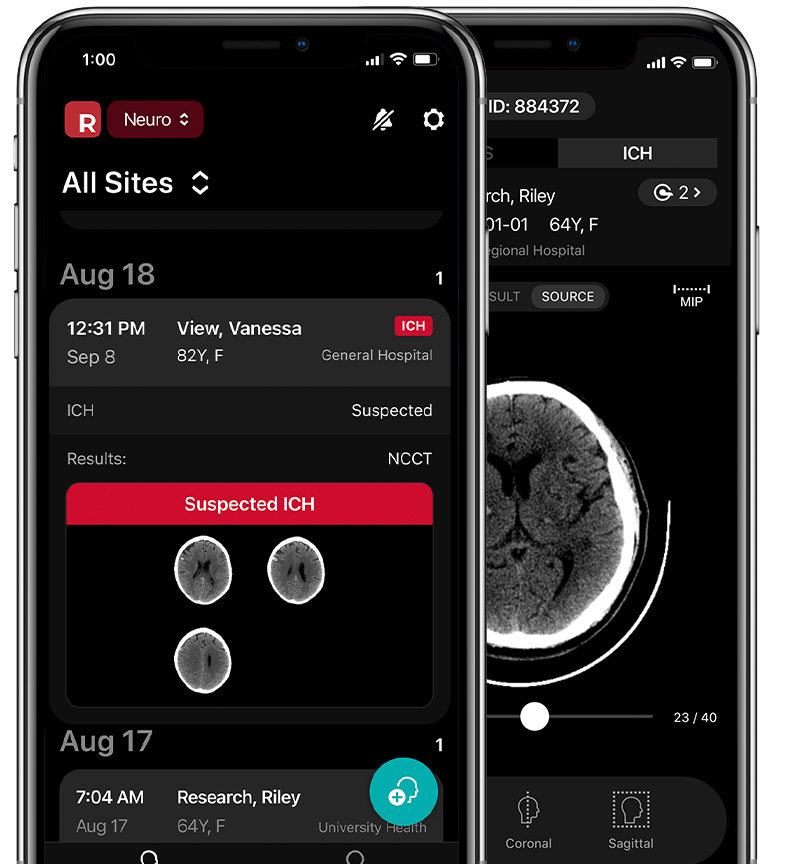
The latest in artificial intelligence for fast, automated detection of intracranial hemorrhage.
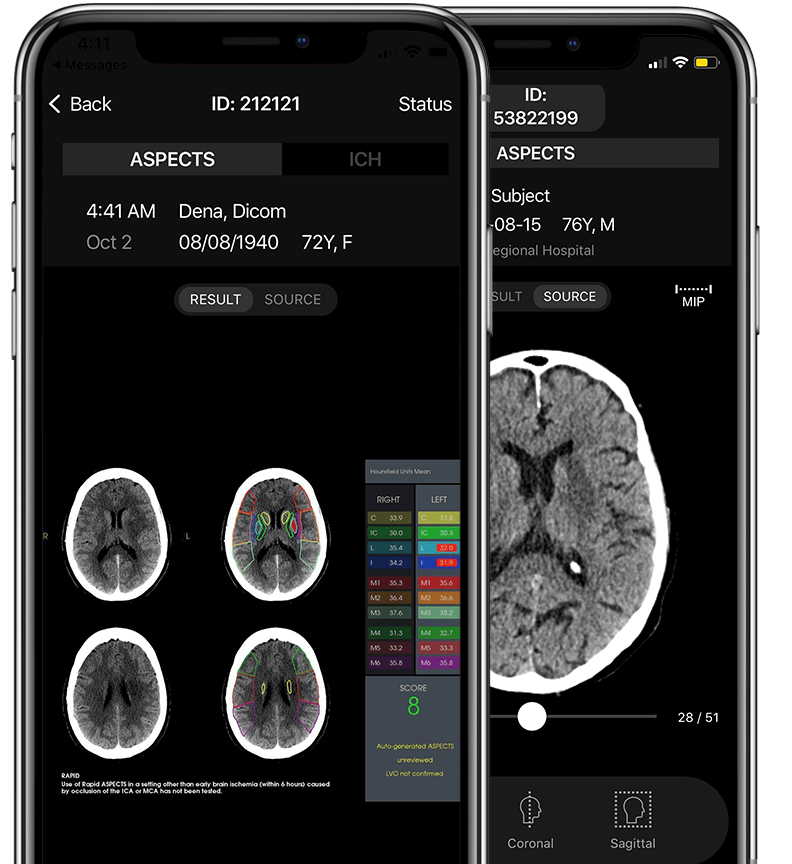
Automatically generates measurements to indicate early ischemic change on NCCT scans.
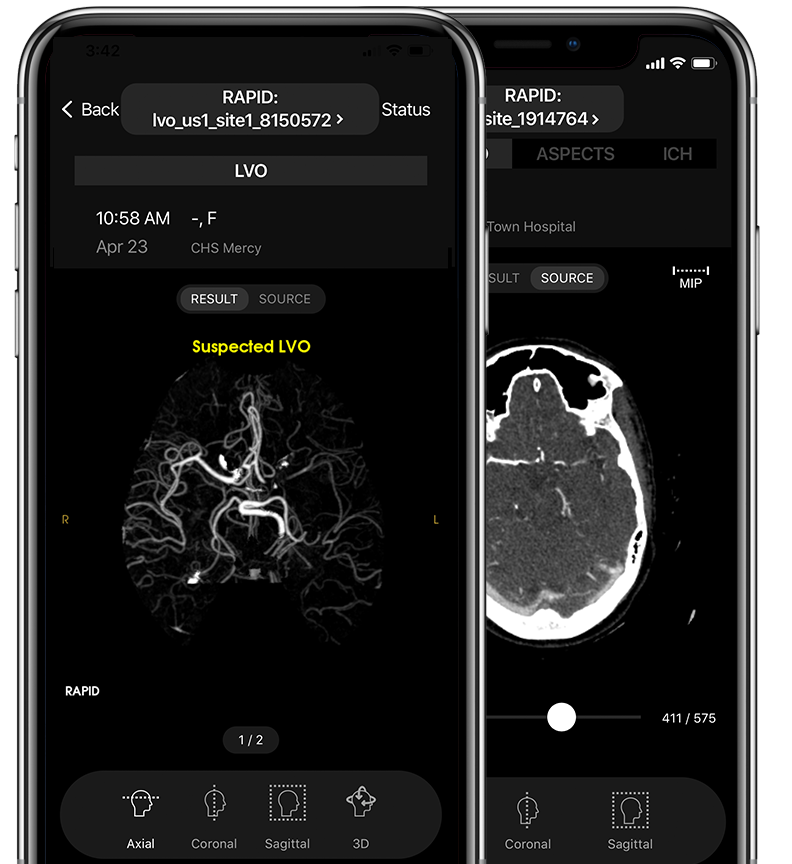
Fast Identification of Suspected LVOs and regions of asymmetry in blood vessel density.
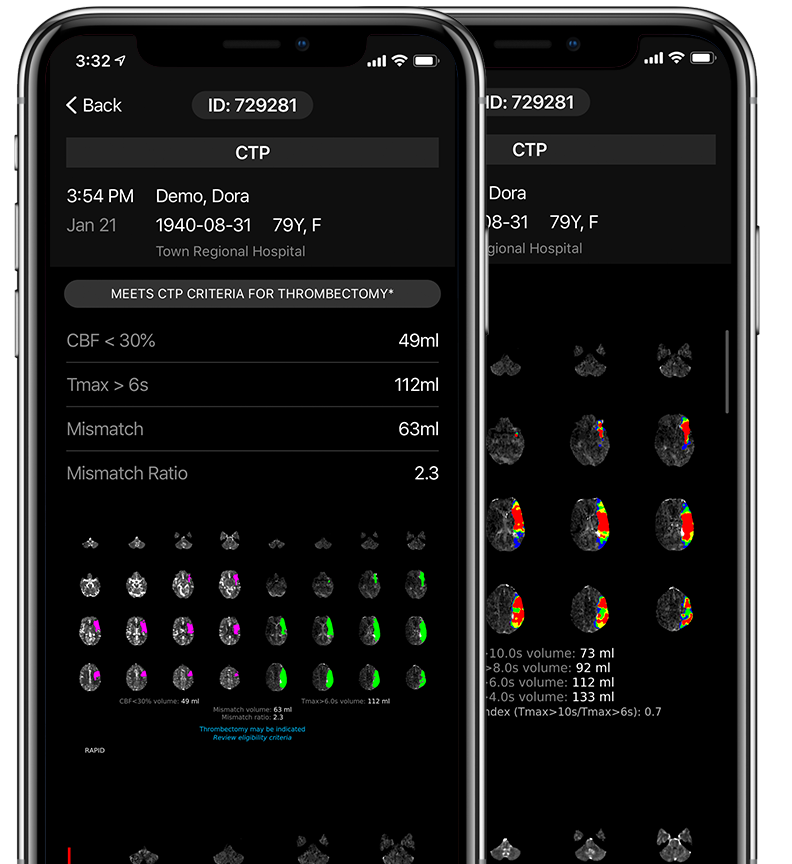
Fast, automated assessment of patients who are likely to benefit from endovascular thrombectomy.
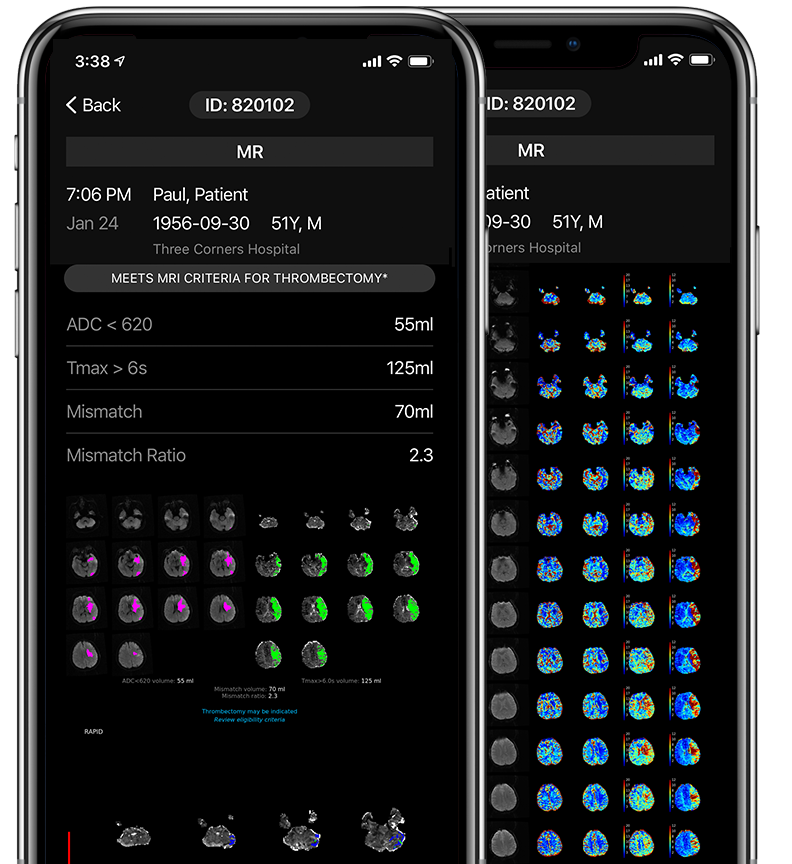
Automatically identifies brain areas with low ADC values and delayed contrast arrival.
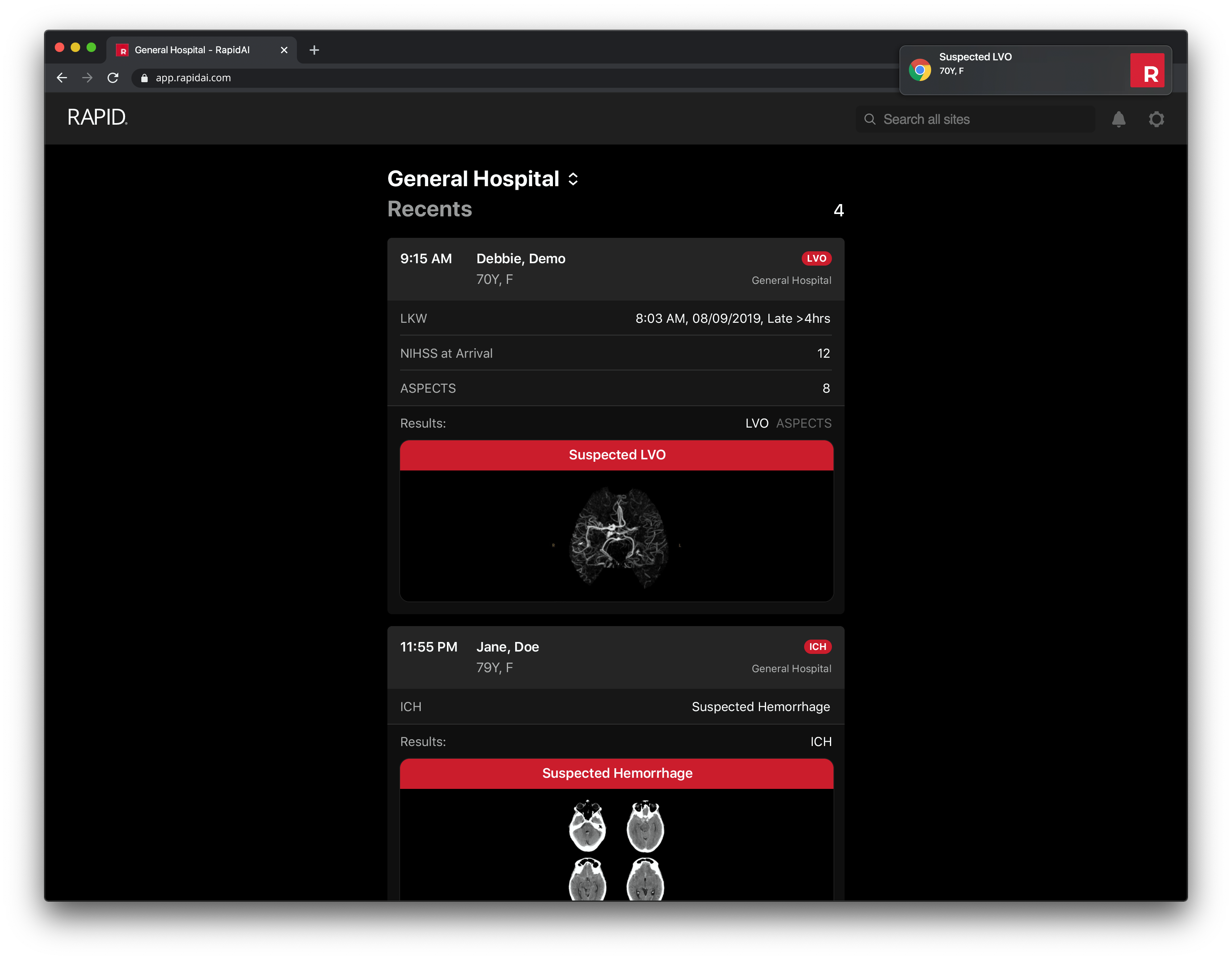
Get notifications for new cases and access Rapid results using Google Chrome or Microsoft Edge on any desktop or laptop — saving you and your patients precious time.
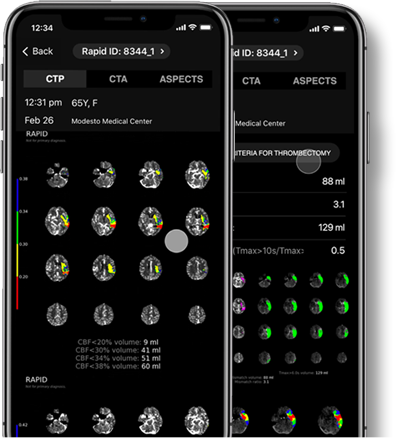
Anytime, anywhere access to RapidAI results plus the ability to communicate about patient care.
“Our research use of Rapid has shown that its speed and accuracy are very helpful in the management of patients with acute stroke. Rapid plays an important role in selecting which patients should be urgently transferred to regional stroke centers for endovascular therapy."
“...We used Rapid in the EXTEND, EXTEND-IA and EXTEND-IA TNK randomized trials at over 20 sites across Australia and New Zealand. It's straightforward to install and works with the full range of CT and MR devices and PACS systems. The fully automated results are robust to common artefacts, easily interpretable and provide standardization across sites with a range of imaging experience.”
"Advanced imaging with Rapid was instrumental in changing the AHA guidelines for thrombectomy (mechanical reperfusion) and has the potential to be equally impactful for thrombolysis (chemical reperfusion)."


